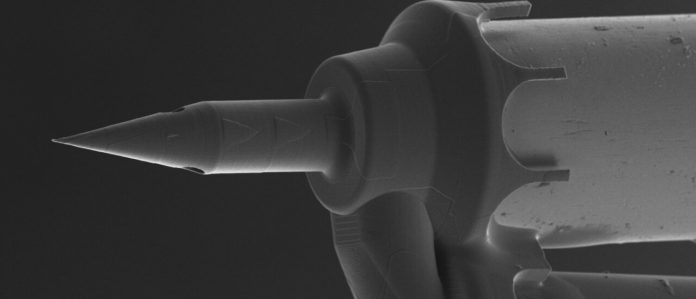
A groundbreaking new microneedle, created by ENT surgeon Dr. Anil Lalwani and mechanical engineer Dr. Jeffrey Kysar from Columbia University, is set to change the way we treat hearing loss and other inner ear conditions.
This needle is thinner than a human hair, sharper than any needle currently on the market, and specifically designed to safely deliver treatments to the delicate inner ear.
The cochlea, a spiral-shaped, fluid-filled structure in the inner ear, is critical for hearing.
But it’s surrounded by the hardest bone in the human body, making it nearly impossible to access safely.
A thin membrane inside the cochlea offers a potential entry point, but traditional surgical tools often tear this fragile tissue, which can cause permanent hearing and balance issues. That’s where the new microneedle comes in.
Dr. Lalwani explains that this microneedle could transform the way doctors deliver gene therapies to the inner ear.
These therapies aim to restore hearing by regenerating damaged cells that we lose due to aging or exposure to loud noise. However, without a precise and safe delivery method, these treatments have been difficult to implement.
The microneedle also has diagnostic potential. By using it to extract fluid from the cochlea, doctors could better understand conditions like Meniere’s disease, which causes dizziness, nausea, and hearing loss.
This could lead to more accurate diagnoses and, eventually, better treatments.
The collaboration between Dr. Lalwani and Dr. Kysar began over 12 years ago, thanks to an introduction by their research team members. Dr. Kysar, intrigued by the challenges of accessing the inner ear, dived into studying its structure and saw the potential for innovation.
Designing the microneedle wasn’t easy. Dr. Kysar explains that the cochlear membrane acts like a tightly stretched tarp: if a hole is too large, it tears.
Their goal was to create a needle sharp and small enough to avoid ripping the membrane.
The solution came through advanced 3D printing technology, allowing them to create needles with tips smaller than 200 nanometers, much thinner and sharper than any commercial alternatives.
The team rigorously tested the microneedle in animal studies, confirming it doesn’t cause hearing loss or permanent damage. The hole it creates heals within two days, making it safe for repeated use. They’ve also demonstrated its potential in delivering treatments, including gene therapy, and using it to visualize the inner ear’s structure via MRI.
In one study, the microneedle successfully delivered a contrast agent into the inner ear of guinea pigs, helping researchers identify changes in the cochlea that could aid in diagnosing Meniere’s disease. Another study showed that the microneedle could deliver gene therapies without harmful side effects.
The team’s next challenge is refining the needle for human use. They’ve designed a dual-lumen version that can simultaneously inject treatments and remove fluid, preventing pressure buildup in the cochlea.
Their company, Haystack Medical, is working to bring this technology to market, collaborating with gene therapy developers to make these treatments a reality.
Dr. Lalwani and Dr. Kysar believe their microneedle could safely allow repeated therapies and open new doors for diagnosing and treating inner ear conditions. With this innovation, the future of hearing loss treatment looks more hopeful than ever.








Leave a Comment