Eating a porkchop. Getting a mosquito bite. Playing with your dog.
Interactions with animals are a common yet significant part of the human experience. While most animal encounters are harmless, some can pose serious threats to human health.
Animals harbor infectious agents called “zoonoses” that can spread to humans and cause severe illness (1). We are most likely to become infected by animals such as pigs, cattle, or rodents that play important roles in our daily lives as food sources or cohabitants of our environments. In recent years, there has been a sharp increase in the number of emerging zoonoses as the demands of the modern world reshape human-animal interactions (2, 3). Changes in land use and habitat destruction have diminished biodiversity and forced many animals into closer contact with humans, increasing interactions that can lead to viral transmission (4). Additionally, global interconnection and the ease of international travel make it much easier for new pathogens to rapidly spread around the world. The recent emergence of zoonoses such as the COVID-19 coronavirus SARS-CoV-2, which is believed to have come from bats, and the monkeypox virus, which originates in rodents, provide particularly salient examples of the effects zoonotic infections on public health (5, 6).
On a biological level, several factors help zoonotic viruses like SARS-CoV-2 jump species boundaries from animals to humans. One is a virus’s ability to enter the human cell. Entry is regulated by interactions between proteins on the surface of the virus and proteins called receptors that are embedded in the cell membrane. The virus also interacts with receptors in the cell membrane of its animal host, so if humans possess the same type of receptors, entry into the human cell is possible. For example, SARS-CoV-2 surface proteins bind to angiotensin-converting enzyme (ACE2), a receptor found in many animals such as sheep, goats, and bats (7). The ACE2 receptor is also found on the surface of human throat and lung cells which allows binding of SARS-CoV-2 surface proteins (8). Viral surface proteins evolve over time and accumulate genetic changes that can improve receptor binding and increase the chances that the virus can enter the cell (9).
But in order to cause respiratory illness, the virus must be able to replicate. To do so, it must evade detection by our innate immune system– the first line of defense against foreign pathogens. The virus and the infected cell engage in an immunological game of tug-of-war to gain control over host cell processes. For example, upon detecting the virus inside the cell, the cell produces small molecules known as interferons that inhibit viral infection. SARS-CoV-2 counters this immune defense by producing a specific protein that can suppress interferon production (10). Viruses that can successfully evade the host immune response can then strongarm the cell to create an environment conducive to viral replication.
Viral diversity is another important factor in zoonotic virus transmission. The more versions of a virus there are, the more likely there will be one that can effectively enter the human cell, evade the immune system and replicate. We can think of the process of virus transmission as a marathon in which the award at the end is successful infection of a new host. Changes to an original virus may grant it better running shoes or higher stamina, helping it reach the finish line.
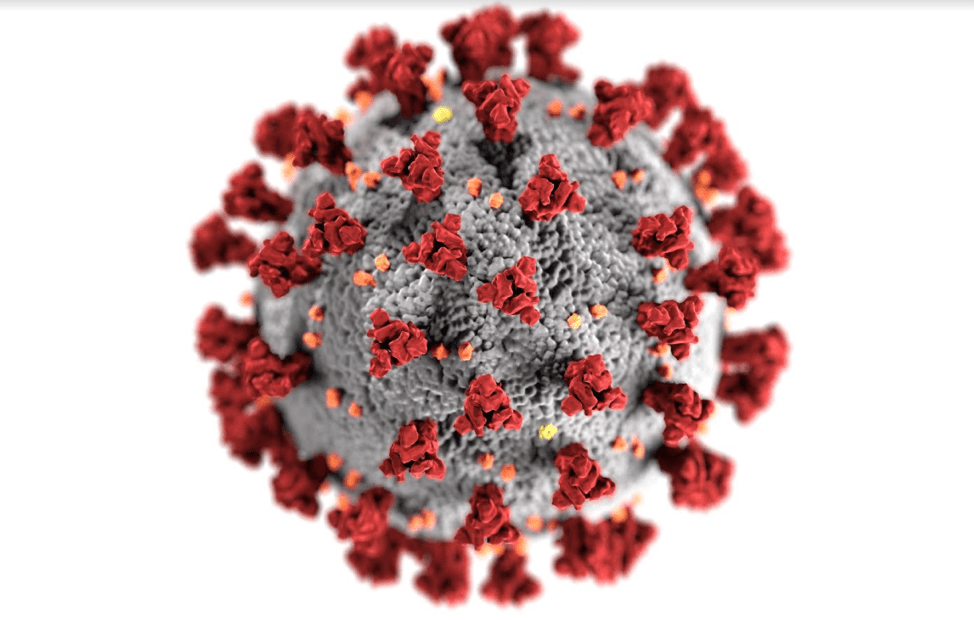
These changes can occur in the form of mutations. Coronaviruses, for example, store their genetic information as RNA and during replication, an enzyme known as an RNA-dependent RNA polymerase generates many new copies of this viral RNA. The enzyme isn’t error-free and may introduce mutations to the RNA sequence during replication (11). Another enzyme known as an exoribonuclease cleaves nucleotides from RNA to help edit the errors introduced during replication, but it also isn’t perfect. (12). The variability in both mutations and how they are edited can create new viral variants to participate in the marathon.
Another source of viral diversity is recombination, which occurs when the RNA polymerase enzyme switches from the original RNA sequence it is copying to a new region of the virus or to an entirely new source of genetic material in the cell (13). Like mutations, recombination can produce a viral variant that has a better chance at overcoming genetic and immunological barriers associated with infecting a new host.
The ability of a virus to jump species boundaries is a complex relationship between viral evolution and genetics and the human immune response. As we continue to navigate the ongoing COVID-19 pandemic, monkeypox outbreak and concerns over the viruses that will inevitably emerge in the future, scientists are developing methods to detect and genetically classify as many viruses as possible and to evaluate their potential to spread and cause disease. (14, 15). These sequence databases will be invaluable tools in efforts to detect existing pathogens and those that have yet to be discovered.
References:
- https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html
- https://www.nature.com/articles/d41586-020-02341-1
- https://www.nature.com/articles/d41586-022-01198-w
- https://www.theatlantic.com/magazine/archive/2020/09/coronavirus-american-failure/614191/
- https://www.science.org/doi/10.1126/science.abh0117
- https://www.who.int/news-room/fact-sheets/detail/monkeypox
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7817217/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7356137/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8167834/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8934133/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8313503/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3744431/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8603903/#CR68
- https://www.nature.com/articles/s41564-022-01089-w
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7656497/
The post Genetic Leapfrog: How Zoonotic Viruses Jump Species appeared first on Illinois Science Council.







Leave a Comment