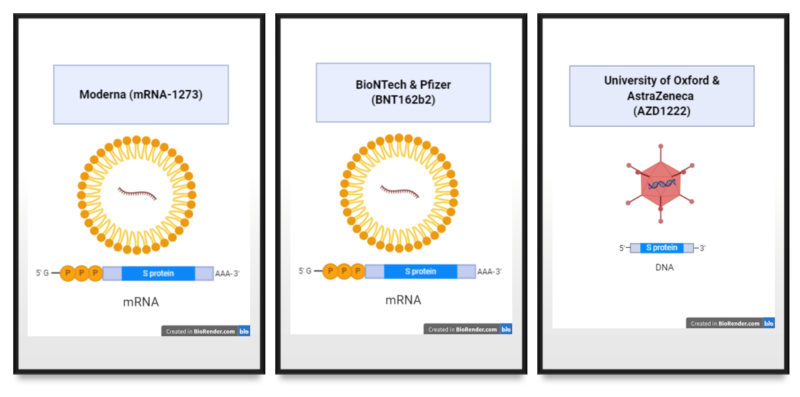
The good news is that we have 3 vaccines that appear to have good efficacy. This means that they protect the people who receive the vaccine. Two of the vaccines are based a new technology that has not been used before in vaccines. Although the technology is new, it is not completely unusual in that it relies on providing instructions (in the form of nucleic acid) for your own cells to make a protein that the virus makes. Other existing vaccine technologies do the same thing, they just provide the instructions in a different form of nucleic acid and delivered in a different kind of “package” (Figure 1).
Pfizer and BioNTech reported that their vaccine was 95% effective at preventing COVID-19 beginning 28 days after the first dose. They enrolled more than 43,000 people in the clinical trial and observed 170 cases of COVID-19. Of those 170 cases, only 8 were in the group that had received the vaccine. This is how they arrived at the 95% effective value. There were 10 severe cases of COVID-19 and 9 of those were the placebo group. Additionally, the vaccine was 94% effective in adults over 65 years old. This information is all coming from a press release, so the results have not be published and peer reviewed yet. The results are positive enough that the companies have applied for emergency use authorization from the FDA.
Limitations are that this vaccine requires 2 doses to achieve the reported efficacy and requires storage and transportation at an extremely low temperature, -70°C (-94°F) to maintain stability. This is good news but not “save the world” news, because of the storage and transportation requirements.
Moderna also reported that their vaccine was 94% effective based on 95 cases of COVID-19 in the more than 30,000 participants in the clinical trial. Among the 95 cases, only 5 were in the group that received the vaccine. All of the cases of severe COVID-19 (11 cases) were in the placebo group. Like the Pfizer and BioNTech vaccine results, these results are coming from a press release. The European Medicines Agency is performing a review of this vaccine as part of the next step to vaccine approval in Europe.
Limitations are that this vaccine requires 2 doses to achieve the reported efficacy and the vaccine requires a higher amount of material than the Pfizer and BioNTech vaccine. However, this vaccine is stable at stable at -20°C (-4°F) for up to six months, at refrigerated conditions for up to 30 days, and at room temperature for up to 12 hours. This is closer to “save the world” news.
These 2 vaccines use mRNA, a type of instructions that cells use to produce proteins. Using the mRNA molecules, cells injected with the vaccine make a protein from the SARS-CoV-2 virus called Spike or S. The S protein is required for the virus to enter into cells. So an immune response mounted against the S protein has a high chance of generating immunity that prevents infection. This hypothesis seems to be true based on the results of the clinical trials with the mRNA-based vaccines.
AstraZeneca also reported positive results with their vaccine. Their vaccine also provides cells instructions for making the S protein from SARS-CoV-2. However, the instructions are packaged inside an engineered virus called chimpanzee adenovirus. This adenovirus cannot replicate and instead is used as a delivery system for the instructions for making S. The advantage to this type of vaccine is it is a DNA vaccine delivered inside a virus particle, which is more stable than an mRNA vaccine delivered inside a lipid nanoparticle (a tiny soap-like bubble) (Figure 1).
AstraZeneca reported results from a study with 23,000 participants who were in one of the following groups: One group received 2 doses of a half dose followed by a full dose, a second received 2 doses of the full-dose amount, and the last received a vaccine against a different pathogen followed by an injection of saline. The different dosing regimens had different efficacies: the half dose followed by a full dose had 90% efficacy and the full dose followed by another full dose had 62% efficacy. Thus, many news reports have stated the average of 70%. Given that the half-dose/full-dose regimen had a better effect and that less material would be required, it seems unlikely that AstraZeneca would proceed with the 2 full-dose regimen. However, it is possible that different groups, like older people, could respond differently and benefit more from the higher dosing regimen.
Among the things we do not know yet are if either of these 3 vaccines prevent asymptomatic disease or spread of the virus. We also do not know how long immunity will last. What we do know is that the vaccines that are in development appear to work and that with combinations of various vaccines, we will reach “save the world” news.
Press Releases
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-submit-emergency-use-authorization
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy
https://investors.modernatx.com/news-releases/news-release-details/european-medicines-agency-begins-rolling-review-modernas-mrna
https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-longer-shelf-life-its-covid-19-vaccine
Clinical trials
Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals
A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19
Phase III Double-blind, Placebo-controlled Study of AZD1222 for the Prevention of COVID-19 in Adults
Science Articles
More People Are Getting COVID-19 Twice, Suggesting Immunity Wanes Quickly in Some
‘Just beautiful’: Another COVID-19 vaccine, from newcomer Moderna, succeeds in large-scale trial
Cover image
Cite as: N. R. Gough, Good News on the COVID-19 Front. BioSerendipity (23 November 2020) https://www.bioserendipity.com/good-news-on-the-covid-19-front/.







Leave a Comment