January 7, 2025
2 min read
Plants’ Photosynthetic Machinery Functions inside Hamster Cells
Transplanted chloroplasts endured two days inside animal cells—and got to work

Researchers transplanted algae chloroplasts into cells from a Chinese hamster (Cricetulus griseus).
Juniors Bildarchiv GmbH/Alamy Stock Photo
More than a billion years ago a hungry cell devoured a tiny blue-green algae. But instead of the former simply digesting the latter, the duo struck a remarkable evolutionary deal. Now scientists are trying to engineer that miracle in a laboratory.
In a recent experiment reported in the Proceedings of the Japan Academy, Series B, researchers transplanted that algae’s photosynthesizing descendants, plant organelles called chloroplasts, into hamster cells—where they converted light into energy, staying active for at least two days.
In 2021 biologist Sachihiro Matsunaga of the University of Tokyo reported how sacoglossan sea slugs can “steal” chloroplasts from algae they eat, fueling the slugs’ energy needs for weeks. His team wanted to recreate this mechanism in other animal cells.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Scientists had previously tried transferring plant chloroplasts into fungi cells, but the cells’ cleanup squad destroyed foreign organelles within hours. For their attempt, Matsunaga’s group harvested extra-hardy chloroplasts from a red algae that thrives in acidic volcanic hot springs and housed them in lab-cultured hamster ovary cells.
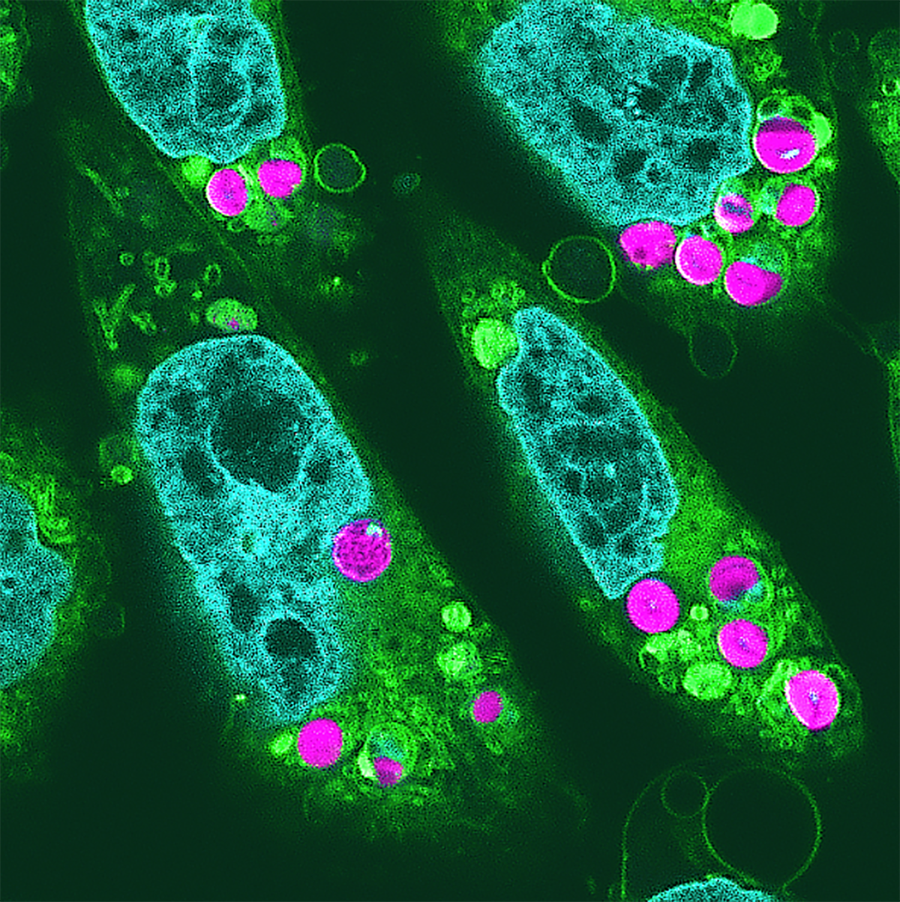
A fluorescence image shows chloroplasts (magenta) successfully incorporated into the hamster cells, with other features of the animal cells also highlighted: nuclei are in light blue, and organelles are in yellow-green.
“Incorporation of Photosynthetically Active Algal Chloroplasts in Cultured Mammalian Cells Towards Photosynthesis in Animals,” by Ryota Aoki et al., in Proceedings of the Japan Academy, Series B, Vol. 100, No. 9; 2024 (CC BY-NC-ND)
The team isolated the chloroplasts from algal cells using a centrifuge and gentle stirring. Instead of then piercing the host cells’ membranes, as in earlier work, the researchers adjusted the culture medium’s composition so it coaxed the animal cells into engulfing the chloroplasts like amoebas do, Matsunaga says, “mistaking them for nutrients.”
The transplanted chloroplasts maintained their structure and showed successful electron transport, a crucial step in processing light, for two days before deteriorating. Previous attempts at transplanting a chloroplast into a foreign cell had worked for just a few hours. “I was impressed that they were able to get that much mileage out of it,” says cell biologist Jef D. Boeke of the NYU Grossman School of Medicine.
Challenges remain: Chloroplasts need a steady supply of proteins from the cell. “Animal cells, however, don’t have the necessary genes to make and transport these proteins, so chloroplasts would break down quickly without them,” says Werner Kühlbrandt, a structural biologist at the Max Planck Institute of Biophysics in Frankfurt. Like Boeke, he was not involved in the new study. Next, Matsunaga’s team plans to try inserting photosynthesis-maintaining genes into animal cells, aiming to make them more compatible with the transplanted chloroplasts.
These types of transplants could someday help scientists engineer living materials, Boeke says, such as photosynthesizing fungi or bacteria that might be used on rooftops to soak up carbon dioxide from the atmosphere, or lab organoids that can grow faster using a chloroplast’s extra oxygen. Solar-powered humans, of course, remain pure fantasy, Matsunaga says: “They would need a tennis court’s worth of surface area covered with chloroplasts.”







Leave a Comment