On a rainy day in July 2024, Tim Bliss and Terje Lømo are in the best of moods, chuckling and joking over brunch, occasionally pounding the table to make a point. They’re at Lømo’s house near Oslo, Norway, where they’ve met to write about the late neuroscientist Per Andersen, in whose lab they conducted groundbreaking experiments more than 50 years ago.
The duo only ever wrote one research paper together, in 1973, but that work is now considered a turning point in the study of learning and memory. Published in the Journal of Physiology, it was the first demonstration that when a neuron — a cell that receives and sends signals throughout the nervous system — signals to another neuron frequently enough, the second neuron will later respond more strongly to new signals, not for just seconds or minutes, but for hours.
It would take decades to fully understand the implications of their research, but Bliss and Lømo had discovered something momentous: a phenomenon called long-term potentiation, or LTP, which researchers now know is fundamental to the brain’s ability to learn and remember. Today, scientists agree that LTP plays a major role in the strengthening of neuronal connections, or synapses, that allow the brain to adjust in response to experience. And growing evidence suggests that LTP may also be crucially involved in a variety of problems, including memory deficits and pain disorders.
Bliss and Lømo never wrote another research article together. In fact, they would soon stop working on LTP — Bliss for about a decade, Lømo for the rest of his life. Although the researchers knew they had discovered something important, at first the paper “didn’t make a big splash,” Bliss says.
By the early 1970s, neuroscientist Eric Kandel had demonstrated that some simple forms of learning can be explained by chemical changes in synapses — at least in a species of sea slug. But scientists didn’t yet know if such findings applied to mammals, or if they could explain more complex and enduring types of learning, such as the formation of memories that may last for years.
The origins of memory
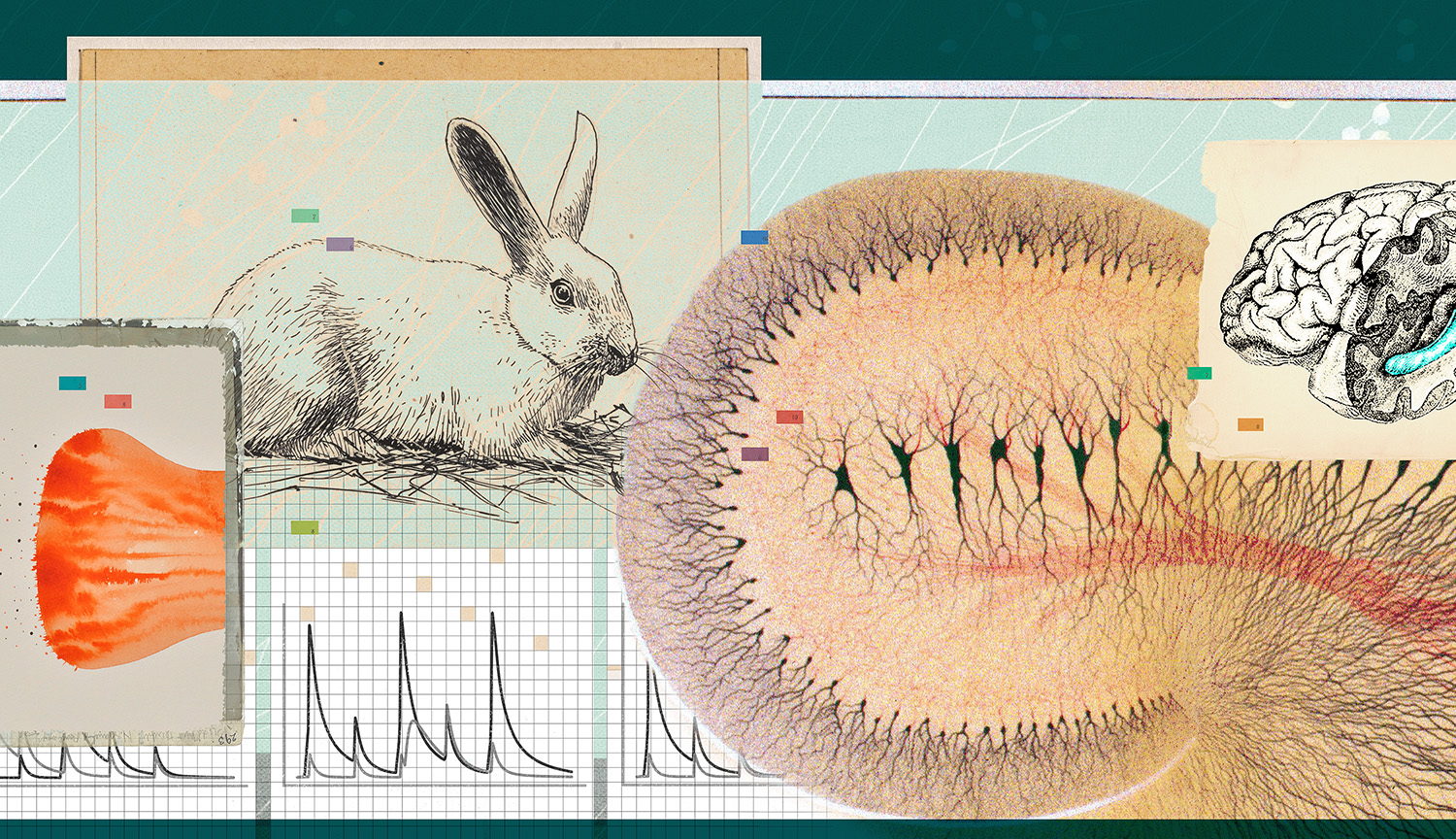
Lømo first discovered LTP while looking for something else. In Oslo, he was studying a brain region called the hippocampus which some evidence suggested was key to storing memories in mammals. Lømo wanted to know if repeated electrical pulses, roughly mimicking neuronal signals, would make hippocampal neurons more sensitive to subsequent stimulation, as previous studies suggested they might. To find out, he delivered timed pulses of electrical current to the neurons in live rabbits. To his surprise, the cells’ responses increased, sometimes for longer than a few minutes. But these long-lasting effects were time-consuming to study, and he had a lot of other work to do.
But then British neuroscientist Tim Bliss arrived in Oslo. During his own PhD studies at McGill University in Canada, he had tried in vain to find a similar phenomenon in the brains of cats. So when he learned of Lømo’s intriguing discovery, he persuaded him to free up one day per week to further explore it.
They used an oscilloscope, which displayed the electrical responses of neurons as a waveform. They would photograph each response for later analysis, which allowed them to compare the activity of a neuron they had left alone to one they had frequently stimulated.
The films were developed at the Institute of Neurophysiology in the heart of Oslo where they both worked, and then hung to dry in the staircase, dangling from the top floor all the way to the basement. Afterward, they would sit at a light box and use paper printed with millimeter-size squares to measure and compare the size of the responses they’d photographed.
The results were unmistakable: After a brief period of high-frequency stimulation, the oscillations would become more pronounced for up to 10 hours, indicating that neurons in the rabbit’s hippocampus responded more strongly — an enduring change that would later become known as long-term potentiation. This looked a lot like the kind of activity many scientists suspected to be at the root of learning and memory.
“Oh, it was fantastic,” says Lømo. “And we were so excited,” Bliss adds. But Bliss and Lømo weren’t ready to publish yet: First, they wanted to better understand their findings. So when they both moved to London to work at different institutions, they continued their weekly get-togethers there. Yet to their dismay, they were unable to recreate their initial results. And when Lømo returned to Oslo in 1971, to try again in the original lab, the experiments didn’t work there either.
After years of pondering, they now agree that the rabbits they used in the second series of experiments were probably stressed. It’s now known that stress enhances LTP in some parts of the hippocampus but suppresses it in others, including the region where Lømo and Bliss measured the neurons’ activity, Lømo says.
Replicating their early results proved so difficult that Lømo decided to move on, to study instead how neurons interact with muscles. Bliss, meanwhile, had some success showing LTP in rabbits that were not sedated during the experiment, but awake, with the electrodes implanted in their brains. It was Bliss’s collaborator on this work, Tony Gardner-Medwin, who pushed for the two studies to be published back-to-back, in 1973. After years of procrastination, Bliss and Lømo published their article.
Learning mechanisms
Early on, the phenomenon that Bliss and Lømo discovered “didn’t really capture the audience that it enjoys now,” says Mark Bear, a neuroscientist at the Massachusetts Institute of Technology. When Bear attended grad school in 1979, he adds, LTP “wasn’t in the textbooks yet.” But a small but growing number of researchers were intrigued.
By the early 1980s, several technological advances had made the study of LTP more tractable. Using slices of hippocampus that could be kept alive outside the brain, for example, researchers could use drugs to block or activate different proteins populating the synapse, to find out how this affected LTP.
The approach revealed that two receptors, proteins specialized in transmitting signals across membranes, on the outside of nerve cells were required for LTP to happen. They were named AMPA and NMDA receptors for the artificial molecules the researchers used to activate them. The work also revealed that the release of a molecule called glutamate from the sending neuron was a crucial step for producing LTP at many synapses in the hippocampus.
Once these key molecules had been identified, scientists started to test whether blocking or enhancing LTP affected learning in lab animals. In one important series of experiments conducted in the 1980s, for example, neuroscientist Richard Morris showed that giving rats a drug that blocks the NMDA receptor impairs their ability to learn how to navigate a maze that untreated rats can easily figure out — and that synapse changes like those seen with LTP occur in the hippocampus when untreated rodents learn.
Still unclear, however, was the sequence of molecular events that induce and maintain LTP, and whether the key changes occur in the neuron that sends the signal or in the receiving cell. Controversy ensued. “Big egos got into the field,” says Bliss. “And big egos go for big questions,” adds Lømo. Bear, who wasn’t involved in this argument, still recalls conversations on a ski lift with LTP researchers at a winter conference. The scientists were “trashing the other guys all the way to the top of the mountain because everybody had a different view of what was going on,” he says.
Hippocampal connections
In the end, both sides turned out to have a point. LTP starts in the receiving neuron, but in most cases, changes in the sending neuron soon follow. Exceptions aside, this is what usually happens at a synapse that gets strengthened with repeated use, at least in a well-studied area of the hippocampus called CA1, which is involved in the formation and retrieval of spatial memories.
First, the sending neuron releases glutamate into the synapse between two neurons. Next, the glutamate molecules bind to the AMPA receptors on the surface of the receiving neuron. When glutamate binds to AMPA receptors, the receptors change shape, opening channels in the membrane that allow sodium ions to flow into the cell.
This influx of sodium ions reduces the electrical difference across the neuron’s membrane, making the inside of the cell less negative compared to the outside — a process called depolarization. At this point, LTP hasn’t happened yet. But if the glutamate release and AMPA receptor activation happen often enough within a certain time frame — 100 times in one second is commonly used to cause LTP in experiments — the resulting depolarization will cause another important receptor on the receiving cell’s surface, the NMDA receptor, to open its own channel.
This allows calcium ions to flow in, which sets in motion a chain of events that will cause the number of AMPA receptors on the receiving neuron’s surface to increase. Meanwhile, the sending neuron boosts the amount of glutamate it stores near its surface. This combination — more glutamate released by the sending neuron, and more AMPA receptors on the receiving neuron — results in a stronger connection between the sending and receiving neurons, an increase which can last for hours, days, even months. And this is what scientists call LTP.
Learning at the synapse
Many questions remain about LTP’s role in learning and memory — especially in humans, who are more difficult to study than rabbits and rats. But Bliss and Lømo’s 1973 study sparked a whole field of research dedicated to understanding the mechanisms of LTP.
Soon after Bliss and Lømo revealed their findings in the 1970s, for example, one pioneer in the field, the late neuroscientist Eva Fifková at the University of Colorado, started studying LTP with electron microscopy, which uses beams of electrons to produce a highly magnified image of an object. “She would rapidly freeze the brain after inducing LTP, then slice it up, make photographs and have them printed out on paper,” explains University of Texas at Austin neuroscientist Kristen Harris.
Fifková was interested in the spines that grow on the tiny treelike protrusions that stud the surface of neurons and allow them to receive signals from other cells. These so-called dendritic spines come in diverse shapes, from mushroom- to thorn-like, and are responsible for making new connections between neurons.
By cutting out spines from the photographs and weighing the resulting paper fragments, Fifková was able to compare the size of spines that were involved in LTP to ones that weren’t. She found that LTP made the spines visibly grow. The next logical question — why? — “inspired my whole career,” Harris says. “I’ve been working on this ever since.”
By creating digital, three-dimensional reconstructions of dendritic spines, Harris and her colleagues have confirmed that LTP indeed causes the dendritic spines to grow. This growth is important, because it creates space inside the cell for the elaborate biochemical machinery that is required to maintain LTP.
If you’re struggling to visualize how synapses work, this video should help.
CREDIT: SALK INSTITUTE
Synapses are often located hundreds of micrometers away from the central part of the neuron where most of the cell’s protein manufacturing occurs. Maintaining LTP requires assembling new, local factories that can build the proteins required for that maintenance, like AMPA receptors. It takes time to produce and assemble the many molecules required for growing and strengthening the synapse, which may explain why we learn best from things we encounter repeatedly, ideally with some time in between, Harris explains. “With every repetition, the connections get stronger.”
While researchers like Harris seek to understand the precise molecular machinery underlying LTP, others have continued to connect this basic research to learning and memory in animals. Bear and his team at MIT, for example, were the first to show that LTP is involved in the formation of fearful memories in mice. In a 2006 experiment, they trained mice to avoid a dark area where they’d previously received an electric shock to the feet. Meanwhile, they used an electrode to record how neurons in the hippocampus responded. “Sure enough, there was LTP,” says Bear: After learning, the rodents’ neurons behaved similarly to neurons experiencing LTP in slices of hippocampus.
When learning hurts
Understanding how LTP contributes to fearful and painful memories could illuminate the causes of anxiety disorders and chronic pain — and potentially point to better treatments. Pain is crucially important for the survival of animals, as a signal of bodily harm. “And it’s a learning experience,” says Michael Salter, a neuroscientist at the Hospital for Sick Children in Toronto. “We put our hand on the stove and think: ‘Ow, I shouldn’t do that again.’
Over the past several decades, researchers have found that LTP isn’t just limited to the hippocampus, but also occurs in other parts of the brain, such as the amygdala, which processes fear, and the cerebral cortex, the part of the brain responsible for perception and reasoning. And though the mechanisms differ, LTP also can occur in other parts of the nervous system, including the spinal cord, Salter adds. “Many people in the pain field,” he says, “would say that LTP in certain brain areas and even the spinal cord may be involved in chronic pain.” Although it’s not “textbook LTP,” he adds, “in the spinal cord, it’s potentiation, for sure, and the NMDA receptor is definitely involved.”
As far as scientists know, some form of LTP can occur in most synapses in the central nervous system. Salter believes that in some cases when pain becomes chronic, it may be caused by LTP in pain-transmitting neurons — a kind of pain that no longer serves its original, protective function. The search is on for a way to eliminate chronic pain without numbing the protective pain we need to stay safe. Still, given the wide-ranging role of the NMDA receptor in coordinating neuronal activity throughout the body, interventions are difficult. The anesthetic ketamine blocks the NMDA receptor, for example, but can have severe side effects. The hope is that finding new ways to target different parts of the NMDA receptor could lead to more precise treatments.
Other scientists are pondering ways of leveraging our understanding of LTP to restore or preserve memory in dementia, reduce anxiety or even enhance learning in all of us. Yet because of the central role of LTP in so many different things that we do, therapies will need to be carefully tested, and the devil will be in the details, says Lømo. “I would expect any really specific treatments to be pretty far into the future.”
The vexing problem of memory loss in dementia, for example, may require a better understanding of the kinds of memories upon which our lives depend, and how LTP contributes to them, Salter says. “What are the precise synapses where LTP occurs that encode such memories? I don’t think we have answered that yet, but I think it’s something we can aspire to.”
Bliss agrees. “The weight of evidence suggests that LTP is central to the physiology of memory storage. But dotting the i’s and crossing the t’s is an ongoing research enterprise.”







Leave a Comment