The pH scale measures how acidic or alkaline a solution is. It ranges between 0 and 14.
Acids have a pH of less than 7.
Alkalis ( or bases ) have a pH of over 7.
pH 7 is neutral.
How is pH measured?
pH is measured using an indicator. An indicator is a dye that changes colour in the presence of an acid or alkali.
Commonly used indicators used to determine pH
- Universal indicator
- Litmus
- Methyl orange
- Phenolphthalein
The image below shows the pH chart for Universal Indicator.
Universal Indicator is a mixture of other indicators.
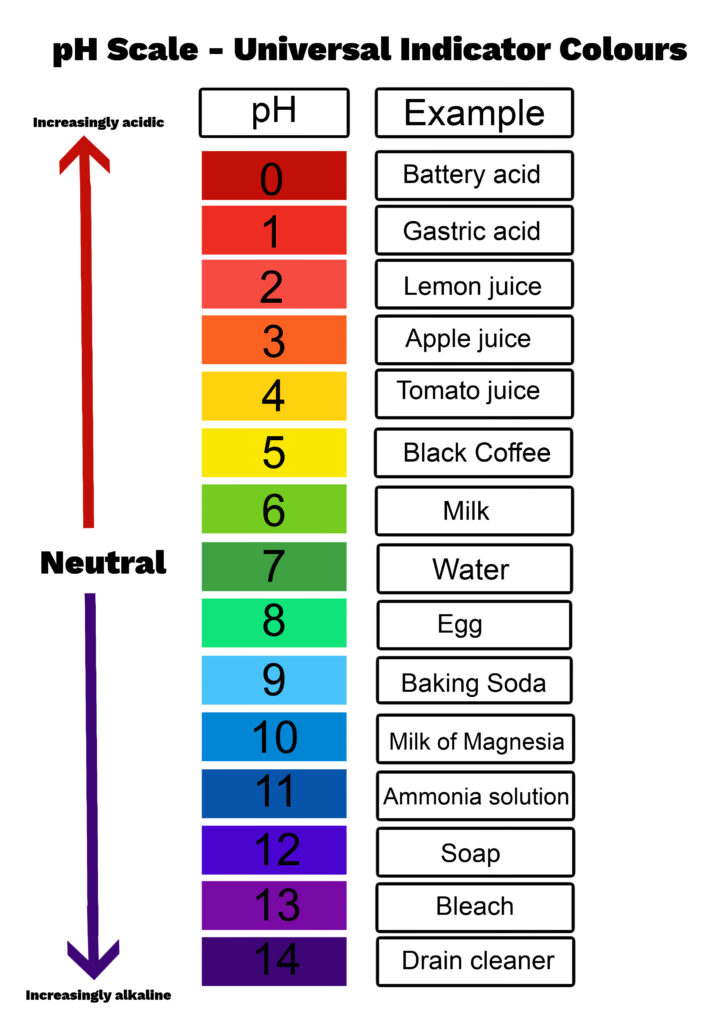
I have a blank version of the image above available for students to use to fill in the example boxes.
Who created the pH scale?
The pH scale was created by a Danish biochemist named Soren Sorensen in 1909.
Simple science experiments for learning about pH
A very basic pH indicator can be made using the liquid left over from boiling red cabbage in water.
The indicator is a purple colour but turns red in the presence of an acid and green if an alkali is added.

A basic indicator can also be made from the leaves of a poinsettia plant!
Last Updated on October 16, 2023 by Emma Vanstone
The post What is the pH Scale? appeared first on Science Experiments for Kids.








Leave a Comment